A device the size of a small paperclip, created to give people with severe paralysis the ability to communicate again, has been shown to be safe.
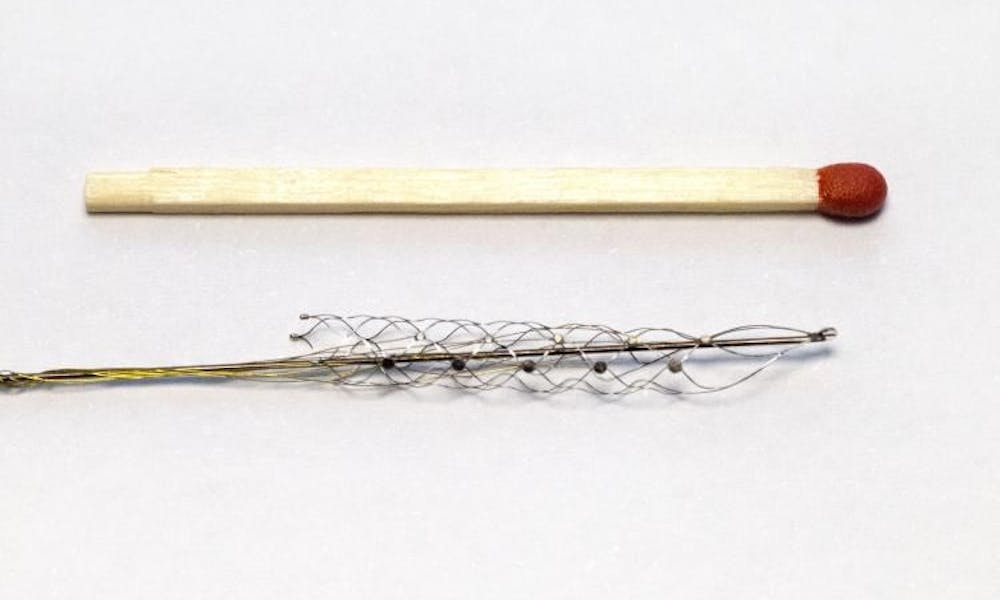
The world first trial has recently concluded and has shown that the tiny device can read brain signals and was safely implanted into all the participants.
The device called the Stentrode™ was placed inside a blood vessel of the brain located in an area that controls movement (motor cortex).
The Stentrode™, was implanted into four participants in the safety trial which was led by the Royal Melbourne Hospital (RMH) and the University of Melbourne.
The trial successfully demonstrated the device could be safely implanted without adverse side effects, and also proved the technology worked to allow patients to generate electrical signals enabling them to do hands-free texting, emailing, online banking and shopping, and communicating care needs using their thoughts.
The research published in JAMA Neurology, was led by the RMH investigators Professor Peter Mitchell, Director, Neurointervention service and Professor Bruce Campbell, Director of Neurology.
Prof Peter Mitchell said the study first and foremost was a safety review of the device implantation.
“The main aim of the study was to confirm the stents remained open with no migration of the device. Signal quality remained stable with no evidence of significant deterioration,” Prof Mitchell said.
All the patients were implanted with the devices in a neurointerventional angiography suite.
“The patients all tolerated the procedure well and were typically discharged home within 48 hours,” Prof Mitchell said.
Prof Bruce Campbell, co-author of the study said it found promising results, even with the limitations of a small study group.
“This technology holds great promise for people with paralysis who want to maintain a level of independence,”
“It was great to work on a trial at the frontier of a new field, and to see the Parkville precinct play a key role shows we are at forefront of emerging technology,” Prof Campbell said.
The trial is continuing in the US with a plan to enrol more participants to continue assessing the safety and efficacy of the device.

We provide a media service from 6am to 9pm each day. Journalists are welcome to contact our media adviser on-call via the RMH Switchboard on (03) 9342 7000.
During business hours, journalists can email mh-communications@mh.org.au. We do not respond to emails outside business hours.

